gravimetric analysis of copper method|precipitation gravimeter chemistry : inc Gravimetric analysis is a quantitative determination of the amount of analyte through a precipitation process, precipitate isolation, and determination of isolated product weight. WEB9 de set. de 2022 · IZA - MOLE (Clipe Oficial) IZA. 4.43M subscribers. Subscribe. Subscribed. 39K. Share. 1.8M views 1 year ago #MOLE #IZA #TRÊS. Ouça #TRÊS nas plataformas: .
{plog:ftitle_list}
web16 de dez. de 2022 · Ranking de Clubes CONMEBOL 2023. CONMEBOL Libertadores. Eng. Esp. Por. Favorite Team . Cambio de equipo.
Gravimetric analysis is a quantitative method for accurately determining the amount of a substance by selective precipitation of the substance from an aqueous solution. The precipitate is separated from the remaining aqueous solution by filtration and is then weighed.In this exercise we will analyze an alloy containing copper to determine its percent content in it. Copper will be precipitated as CuSCN (solubility product Kso=12.7). This means that Cu2+ .Gravimetric analysis is a quantitative determination of the amount of analyte through a precipitation process, precipitate isolation, and determination of isolated product weight. Understanding the conditions that favor particle growth is important when we design a gravimetric method of analysis. We define a solute’s relative supersaturation , RSS , .
Gravimetric Methods of Analysis [gravi – metric; weighing – measure] Hydrated and anhydrous copper sulfate [CuSO4 xH2O] Features of Gravimetric Analysis •A given analyte is isolated .
Gravimetric determination of copper as CuSCN Gravimetric analyses belong to the most precise, because contemporary analytical balances make possible . This is an excellent method, since most thiocyanates of other metals are soluble, in particular thiocyanates of Bi, Cd, As, Sb, Sn, Fe, Ni, Co, Mn and Zn. In presence of moderate amounts of Bi .12A-1 Properties of Precipitates and Precipitating Reagents A gravimetric precipitating agent should react specifically or at least selectively with the analyte and give precipitates that is: 1. Enough particle size for retaining on filter paper 2. High purity (free of contaminants) 3. Low solubility that no significant loss of the analyte occurs during filtrationcontent by re assay using gravimetric method based had a precision value (% RSD) was 3.3850%, while the value . gold concentration and the further method to nal determination. Basically there are three methods used for determining of gold likely . 30% copper, but also contains high grade gold and silver. The gold content varies between 20 .Questions on Sulfate Analysis. Approximately how many mL of 5% BaCl 2 2H 2 O solution would be required to precipitate all the sulfate if we assume that your samples are pure sodium sulfate? Assume that the density of the barium chloride solution is 1.00 g/mL. If the samples were pure potassium sulfate would you require a smaller or larger volume of barium chloride solution .
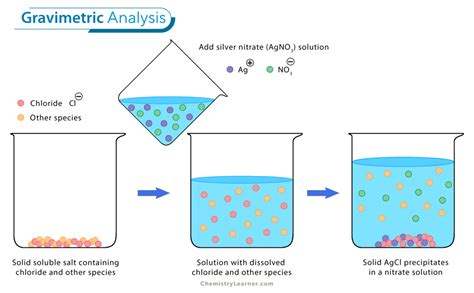
established analytical methods we consider this term. Precipitation Gravimetry Gravimetric analysis is a standard classical method for determining the amount of a given component present in a host of solid and solution sample types. The method used here involves precipitating the component of interest from the unknown by means of an added reagent.analysis is performed, it is almost impossible to prevent photodecomposition of the wet silver chloride precipitate. Generally, the effect is small and can be ignored . Gravimetric Chloride Unknown #88 T.A. Lee Your name goes here, not mine! 1st . Method 925.10 in Official Methods of Analysis, 18th Edition (AOAC International, 2007) provides an approved method for determining the moisture content of flour. A preweighed sample is heated for one hour in a 130 o C oven and transferred to a desiccator while it cools to room temperature. Standard Test Methods for Chemical Analysis of Copper Alloys E0478-08R17 ASTM . Copper by the Combined Electrodeposition Gravimetric and Oxalyldihydrazide Spectrophotometric Test Method [50 %, minimum] 10 – 18. Iron by the 1,10-Phenanthroline Spectrophotometric Test Method [0.003 % to 1.25 %]
ford v10 compression tester
Gravimetric analysis is based on mass measurement. Q-11: What is the gravimetric analysis principle? Answer: Gravimetric analysis is based on the difference in masses of two analyte-containing compounds. The idea behind gravimetric analysis is that the mass of an ion in a pure compound can be calculated and then used to calculate the mass . The growth kinetics of copper microparticles was analysed by using the gravimetric method. The copper microparticles were synthesized in aqueous solution containing cupric ion and HCHO under various conditions (temperature, additive) and the total mass was monitored during the synthesis. The relation between the toThe growth kinetics of copper microparticles was analysed by using the gravimetric method. The copper microparticles were synthesized in aqueous solution containing cupric ion and HCHO under various conditions (temperature, additive) and the total mass was monitored during the synthesis. . analysis. The gravimetric method is one of the oldest .
Gravimetric analysis is an analytical technique used for the quantitative determination of an analyte based on the mass of a solid. . Electro gravimetric method is employed to separate the ions of a substance, often a metal. In this method, the analyte solution is electrolyzed. As a result of the electrolytic reduction, the analyte is .Learn about gravimetric analysis, a method to determine the amount of a substance by measuring its mass. Analytical Chemistry, Volume 7: Gravimetric Analysis, Part II describes the experimental procedures for the gravimetric analysis of Groups I to V cations. This book is composed of 43 chapters that also present sample preparation, separation, and precipitation protocols. The first six chapters include Group I cations, such as silver, lead, mercury, copper, .
PDF | On Jan 1, 2018, Dallatu E. Musa and others published Electrogravimetric Determination of Copper Using a Constructed Compact Electrolytic Cell | Find, read and cite all the research you need .
Gravimetric analysis can be used to determine the concentration of an unknown chloride solution or the percentage by mass of an unknown chloride salt. A common method is to add an excess of acidified silver nitrate to a solution of the unknown salt to form a silver chloride .COPPER BY THE COMBINED ELECTRODEPOSITION GRAVIMETRIC AND OXALYLDIHYDRAZIDE SPECTROPHOTOMETRIC TEST METHOD 10. Scope 10.1 This test method covers the determination of copper in compositions greater than 50 %. 10.2 This international standard was developed in accor-dance with internationally recognized principles .Decomposition Method" at the end of this document) Figure 1. Microwave carousel and vessels. The lid torque tool is labeled A. NOTE: Label tape must NOT be applied directly to the Teflon vessel, but should be used on the fiber vessel sleeves. 1. Accurately weigh three 1.0 to 1.2-g samples of the dried unknown into clean, dry Teflon PFA A good example of this type of gravimetric analysis is the determination of the concentration of copper ions in a solution. Thermogravimetric Method In thermogravimetric analysis, the thermal stability of materials is studied by measuring the changes in their mass as they are subjected to controlled heating.
03.2 The calorimetric methods for bismuth and iron given in IS.: 440-1955 have been replaced by the photometric methods. 0.33 The methods for determination of copper, tin, nickel, arsenic, antimony and lead given in IS : 440-1955 have been revised. 0.4 The methods of analysis prescribed in this standard have been prepared with a view that they .The bubbling was due to the production of CO 2.. The test of vinegar with potassium carbonate is one type of quantitative analysis —the determination of the amount or concentration of a substance in a sample. In the analysis of vinegar, the concentration of the solute (acetic acid) was determined from the amount of reactant that combined with the solute present in a known .Gravimetric analysis is a quantitative method used in analytical chemistry to determine the amount of a substance present in a sample by measuring its mass. This technique relies on the principles of precipitation and weighing to isolate and quantify the analyte of interest. This method is often used in environmental monitoring, pharmaceutical .
silver ion precipitation gravimetry
precipitation gravimetry solutions
Hence, w g of copper(1) thiocyanate = 121.62. g of copper(I1) sulphate. This much copper(I1) sulphate is present in 50 cm3 of test solution. Hence, concentration of copper(I1) sulphate in test solution Concentration of CuS0,.5H20 = 249.5~ 1000x mass of CuSCN 121.62~ volume of test solution g dm-3 Calculations for zinc7 Gravimetric Analysis Dr. Meenu. Gravimetric Analysis; On macroscopic level gravimetric analysis is based on obtaining easily filterable precipitates that are free from contaminants. The process which involves conversion of analyte into sparingly soluble compounds of known composition, filtered, washed and dried is precipitation gravimetric . Activity 2: Gravimetric determination of the formula of hydrated copper(II) sulfate. Gravimetric analysis to determine the moles of water present in a hydrated complex usually requires preweighing of a sample and heating to constant mass over a Bunsen burner using a crucible and a desiccator to prevent water from being reabsorbed from the air.
For example, one standard gravimetric method for the determination of magnesium involves its precipitation as MgNH 4 PO 4 •6H 2 O. Unfortunately, this precipitate is difficult to dry at lower temperatures without losing an inconsistent amount of hydrated water and ammonia. . A 0.7336-g sample of an alloy containing copper and zinc is .The basic reaction in the determination of copper using the iodometric method is represented by the equation: \[2Cu^{2+} + 4I^- \rightleftharpoons 2CuI(s) + I_2\] This is a rapid, quantitative reaction in slightly acidic solutions, if there is a large excess of iodide ion present and if the copper is in the form of a simple ion rather than a .
forearm compression test
Build the Ultimate Team and challenge friends in a FIFA Mobile football game for iOS and Android! Get it on Apple App Store or Google Play.
gravimetric analysis of copper method|precipitation gravimeter chemistry